HYRNUO WAS EVALUATED IN NSCLC PATIENTS WITH HER2 TKD ACTIVATING MUTATIONS¹
71% OF PATIENTS TREATED WITH HYRNUO ACHIEVED AN OBJECTIVE RESPONSE1†‡
(95% CI: 59, 82; N=70)
CR: 2.9%, PR: 69%
MEDIAN DoR OF HYRNUO WAS 9.2 MONTHS1†§
(95% CI: 6.3, 15.0; N=50)
†Major efficacy outcomes were confirmed ORR and DoR as assessed by BICR using RECIST v1.1.
‡ORR (95% CI) calculated using the Clopper-Pearson method.
§Kaplan-Meier estimate.
CR=complete response; ORR=objective response rate; PR=partial response.
HYRNUO SAFETY PROFILE
¶Safety was evaluated in 136 patients who had received prior systemic therapy but were naive to HER2-targeted treatment, and patients who had received prior systemic therapy including HER2-targeted ADC.
ADVERSE REACTIONS IN THE SAFETY POPULATION WERE MOSTLY LOW GRADE AND MANAGEABLE1
- HYRNUO was associated with mostly Grade 1-2 adverse reactions, with no Grade 4 events reported except for dyspnea (0.7%)
- The most common adverse reactions (>20%) in patients who received HYRNUO were diarrhea (87%), rash (66%), paronychia (33%), stomatitis (29%), and nausea (21%)
- Most diarrhea adverse reactions were low grade (1-2) and did not result in treatment discontinuation
- Grade 3-4 adverse reactions included diarrhea (18%), decreased appetite (2.9%), vomiting (2.2%), stomatitis (1.5%), nausea (1.5%), rash (1.5%), pruritus (1.5%), dyspnea (1.5%), decreased weight (0.7%), fatigue (0.7%), and ocular toxicity (0.7%)
- The most common Grade 3-4 laboratory abnormalities (≥2%) were decreased potassium (13%), increased lipase (12%), decreased lymphocyte count (6%), decreased sodium (4.4%), increased amylase (3.8%), increased AST (3%), and increased ALT (3%)
- Serious adverse reactions occurred in 31% of patients
- Most common (≥2%): diarrhea (6%), pneumonia (3.7%), dyspnea (2.2%), pleural effusion (2.2%)
- These are not all the possible side effects of HYRNUO
MOST PATIENTS CONTINUED HYRNUO WITHOUT DOSE
REDUCTION OR INTERRUPTION1
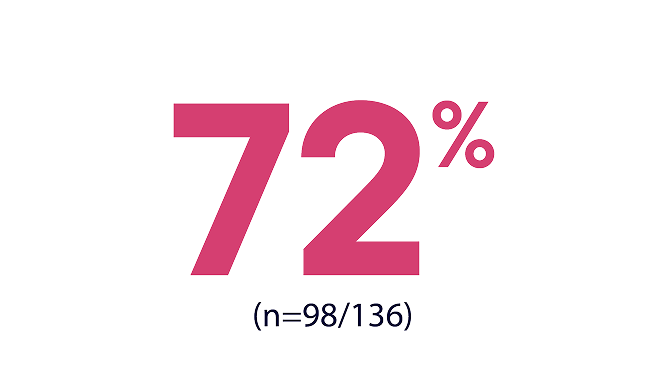
WERE TREATED WITH NO DOSE REDUCTIONS1
Dose reductions due to adverse reactions occurred in 28% of patients who received HYRNUO
diarrhea
rash
hypokalemia

CONTINUED TREATMENT WITHOUT INTERRUPTION1
Dose interruptions due to adverse reactions occurred in 46% of patients who received HYRNUO
diarrhea
hypokalemia
nausea
decreased appetite
pneumonia
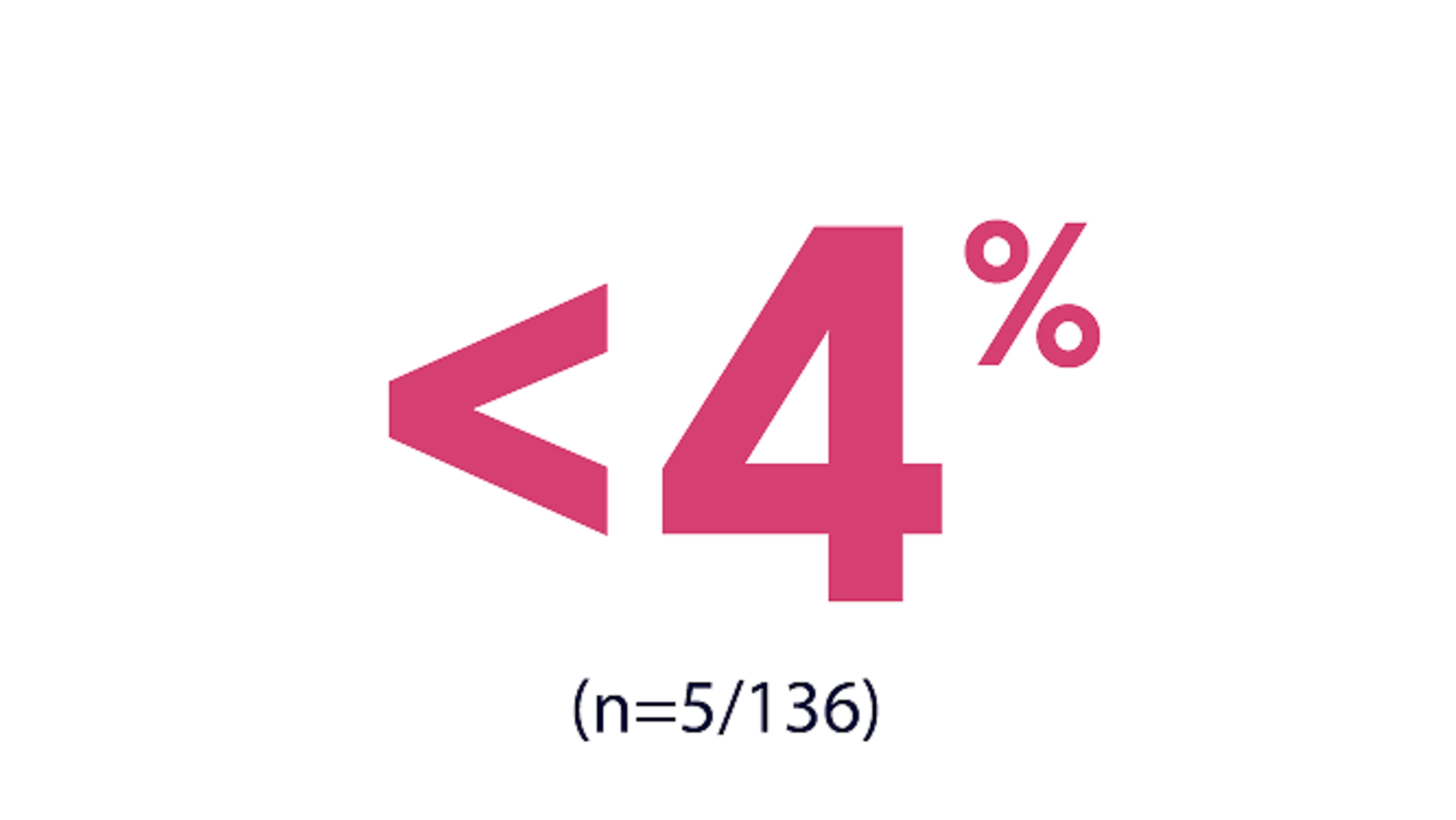
DISCONTINUED TREATMENT DUE TO ADVERSE REACTIONS1
corneal epithelial microcysts
hepatic function abnormal
electrocardiogram QT prolonged
pain in extremity
dyspnea
RECOMMENDED DOSING OF HYRNUO1

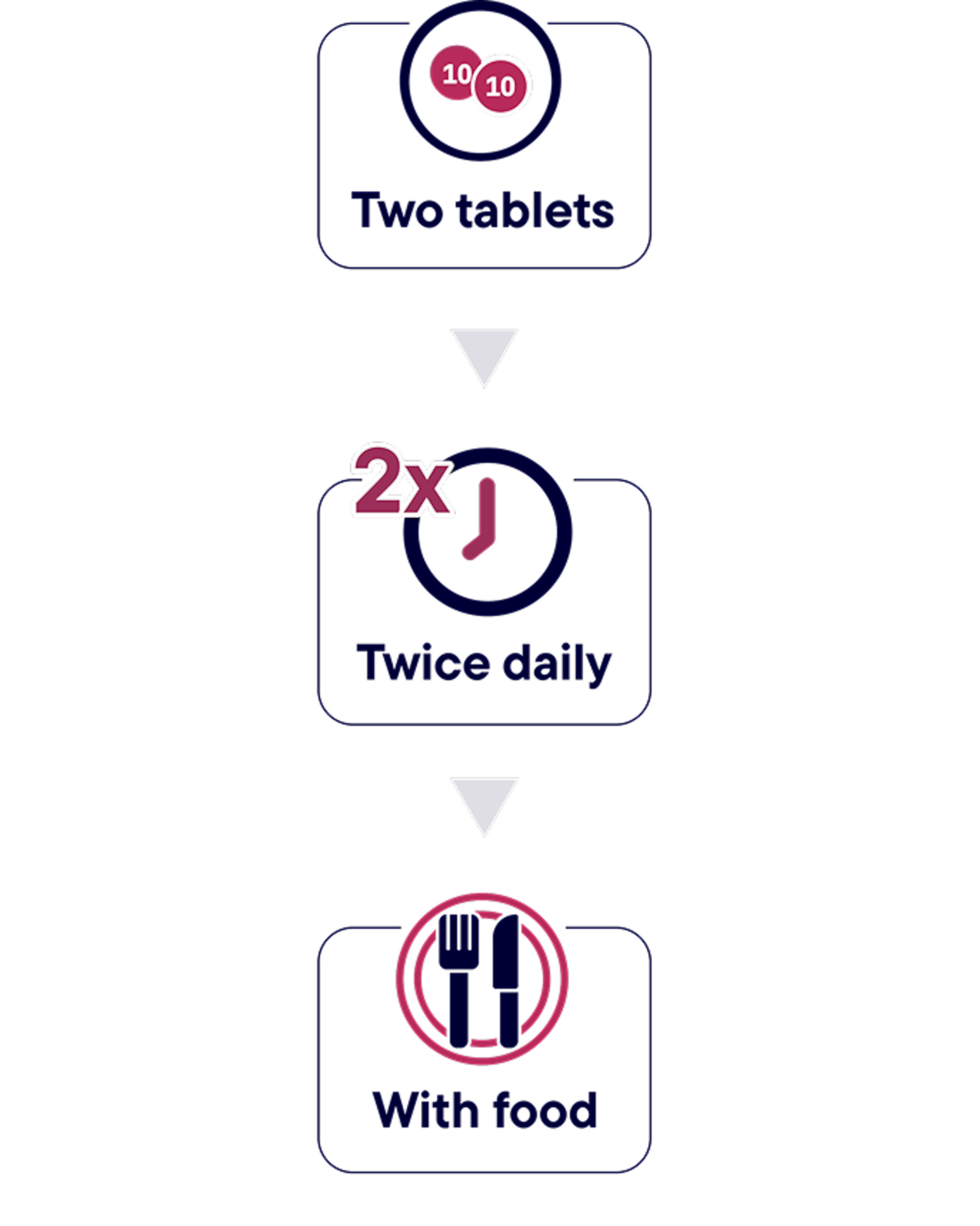
No weight-based dosing is required.
The recommended dose is 20 mg (two 10 mg tablets) orally twice daily with food, for a total daily dose of 40 mg, until disease progression or unacceptable toxicity. Swallow tablets whole. Do not cut, crush, or chew tablets.
If a dose is missed, patients must take the missed dose as soon as they remember prior to the next scheduled dose. Patients should not take 2 doses at the same time to make up for a missed dose.
EXPLORE HELPFUL MATERIALS
TO RECEIVE A PATIENT STARTER KIT, VISIT THE REQUEST A
SPECIALIST PAGE.
COMPREHENSIVE SUPPORT THROUGHOUT YOUR PATIENTS’ TREATMENT JOURNEY
Once you've made the decision to prescribe HYRNUO, you can do so through our dedicated specialty pharmacy, Onco360TM. Acting as an extension of your office, Onco360TM provides oncology-focused care to your patients.
FOR ALL PATIENTS
- One-month supply of HYRNUO allows HCPs and patients to determine if it is the right treatment option at no cost to payer or patient. Enroll through a Bayer-contracted specialty pharmacy
FOR PATIENTS WITH MEDICARE
- Ask patients with limited income and resources to check their eligibility for government-funded programs, such as Medicare Part D Extra Help, etc, at www.medicare.gov/basics/costs
- Patients can opt in to the Medicare Prescription Payment Plan to help manage out-of-pocket drug costs by spreading them out over the year
*Participation in the HYRNUO Free Trial Program is limited to 1 time only per product (patients currently using HYRNUO are not eligible for a Free Trial of their current product). The Free Trial Program includes 1 month supply. The Free Trial Program for HYRNUO is available to patients 18 years of age and older. Bayer reserves the right to rescind, revoke, or amend this offer without notice at any time.
†Patients are eligible if they are commercially insured and may pay as little as $0 per month. Benefit limitations apply. Patients who are enrolled in any type of government insurance or reimbursement programs are not eligible. As a condition precedent of the co-payment support provided under this program, eg, Co-Pay refunds, participating patients and pharmacies are obligated to inform insurance companies and third-party payers of any benefits they receive and the value of this program, and may not participate if this program is prohibited by or conflicts with their private insurance policy, as required by contract or otherwise. Void where prohibited by law, taxed, or restricted. Patients enrolled in the Bayer US Patient Assistance Foundation are not eligible. Bayer may determine eligibility, monitor participation, equitably distribute product and modify or discontinue any aspect of the HYRNUO $0 Co-Pay Program at any time, including but not limited to this commercial Co-Pay assistance program.

IF YOUR PATIENTS CANNOT AFFORD THEIR PRESCRIPTION MEDICATION, BAYER MAY BE ABLE TO HELP
The Bayer US Patient Assistance Foundation is a charitable organization that helps eligible patients get their Bayer prescription medicine at no cost. Please have your patient contact the program at